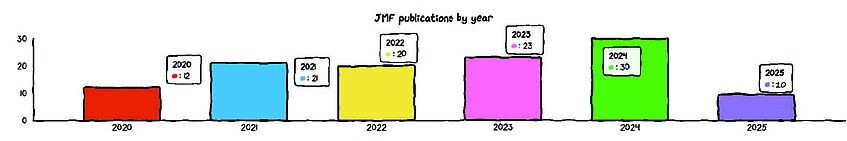
Our Publications in Figures
Peer-reviewed publications we contributed to in the past five years
Where the publications we contributed to get published

List of JMF Publications
2025
Selective heterotopic bacteria can selfishly process polysaccharides in freshwater lakes.
Čačković A, Pjevac P, Orlić S, Reintjes G. 2025 - Cell Rep. 115415.
Sarcletti F, Dijmarescu M, Eigenschink M, Wukowits N, Oehler B, Mayer T, Pell S, Tandecki A, Seki D, Spittler A, Berry D, Berger A, Wisgrill L. 2025 - Eur J Immunol. 55:e202451538.
Peritrophic matrix: an important determinant of vector competence in hematophagous arthropods.
Hodžić A, Duscher GG, Alić A, Beck R, Berry D. 2025 - Trends Parasitol. S1471-4922(25)00069-8.
Nussbaumer-Pröll, A., Hausmann, B., Weber, M., Pjevac, P., Berry, D., Zeitlinger, M. 2025- Antibiotics, 14: 278.
Bao Y, Osowiecka M, Ott C, Tziraki V, Meusburger L, Blaßnig C, Krivda D, Pjevac P, Séneca J, Strauss M, Steffen C, Heck V, Aygün S, Duszka K, Doppelmayer K, Grune T, Pignitter M. 2025 - Redox Biol 81:103575.
Vignolle A, Zehl M, Kirkegaard RH, Vignolle GA, Zotchev SB. 2025 - ACS Omega 10:7163-7171.
Flanagan K, Gassner K, Lang M, Ozelyte J, Hausmann B, Crepaz D, Pjevac P, Gasche C, Berry D, Vesely C, Pereira FC. 2025 - mBio 16:3.
Testing the Purity of Limnospira fusiformis Cultures After Axenicity Treatments.
Schagerl M, Kaptejna A, Polz F, Ali SS, Huo S, Seneca J, Pjevac P, Hechtl V. 2025 - Cells 14:136.
Wittlinger J-P, Castejón N, Hausmann B, Berry D, Schnorr SL. 2025 - Appl Environ Microbiol 91:2.
2024
The Parkinson's disease drug entacapone disrupts gut microbiome homoeostasis via iron sequestration.
Pereira FC, Ge X, Kristensen JM, Kirkegaard RH, Maritsch K, Szamosvári D, Imminger S, Seki D, Shazzad JB, Zhu Y, Decorte M, Hausmann B, Berry D, Wasmund K, Schintlmeister A, Böttcher T, Cheng JX, Wagner M.
2024 - Nat Microbiol 9, 3165–3183.
Deehan EC, Zhang Z, Nguyen NK, Perez-Muñoz ME, Cole J, Riva A, Berry D, Prado CM, Walter J. 2024 - Gut Microbes, 16(1):2363021
Tailless and filamentous prophages are predominant in marine Vibrio.
Steensen K, Séneca J, Bartlau N, Yu XA, Hussain FA, Polz MF. 2024 - ISME J 18:wrae202.
A respiro-fermentative strategy to survive nanoxia in Acidobacterium capsulatum.
Trojan D, García-Robledo E, Hausmann B, Revsbech NP, Woebken D,
Eichorst, SA. 2024 - FEMS Microbiology Ecology, 100: fiae15.
Sulfur-oxidizing symbionts colonize the digestive tract of their Lucinid hosts
Alcaraz CM, Séneca J, Kunert M, Pree C, Sudo M, Petersen JM. 2024 – ISME J :wrae200.
Inhibition of ATGL alleviates MASH via impaired PPARα signalling that favours hydrophilic bile acid composition in mice
Dixon ED, Claudel T, Nardo AD, Riva A, Fuchs C, Mlitz V, Busslinger G, Schnarnagl H, Stojakovic T, Senéca J, Hinteregger H, Grabner GF, Kratky D, Verkade H, Zimmermann R, Haemmerle G, Trauner M. J. 2024 – Hepatol :S0168-8278(24)02577-7.
Genomes and secondary metabolomes of Streptomyces spp. isolated from Leontopodium nivale ssp. alpinum
Malfent F, Zehl M, Kirkegaard RH, Oberhofer M, Zotchev SB. 2024 – Front Microbiol. 15:1408479.
Effect of oral multispecies probiotic on wound healing, periodontitis and quality of life on patients with diabetes
Stuermer EK, Bang C, Giessler A, Smeets R, Janke TM, Seki FD, Debus ES, Franke A, Augustin M. 2024 – J Wound Care. 33:394-407.
Gut microbiota genome features associated with brain injury in extremely premature infants
Seki D, Kirkegaard R, Osvatic J, Hausmann B, Séneca J, Pjevac P, Berger A, J Hall L, Wisgrill L, Berry D. 2024 – Gut Microbes 16:2410479.
Distinct microbial communities are linked to organic matter properties in millimetre-sized soil aggregates
Simon E, Guseva K, Darcy S, Alteio L, Pjevac P, Schmidt H, Jenab K, Ranits C, Kaiser C. 2024 – ISME J :wrae156.
Archaea influence composition of endoscopically visible ileocolonic biofilms
Orgler E, Baumgartner M, Duller S, Kumptisch C, Hausmann B, Moser D, Khare V, Lang M, Köcher T, Frick A, Muttenthaler M. 2024 – Gut Microbes 16:2359500.
Soil cover shapes organic matter pools and microbial communities in soils of maritime Antarctica
Martin V, Schmidt H, Canarni A, Koranda M, Hausmann B, Müller CW, Richter A. 2024 – Geoderma 446:116894.
Plant roots affect free-living diazotroph communities in temperate grassland soils despite decades of fertilization
Dietrich M, Panhölzl C, Angel R, Giguere AT, Randi D, Hausmann B, Herbold CW, Pötsch EM, Schaumberger A, Eichorst SA, Woebken D. 2024 – Commun Biol 7:846.
Dynamics and drivers of fungal communities in a multipartite ant-plant association
Barrajon-Santos V, Nepel M, Hausmann B, Voglmayr H, Woebken D, Mayer VE. 2024 – BMC biol 22: 112.
SoxY gene family expansion underpins adaptation to diverse hosts and environments in symbiotic sulfide oxidizers
Sudo M, Osvatic J, Taylor JD, Dufour SC, Prathep A, Wilkins LG, Rattei T, Yuen B, Petersen JM. – Msystems:e01135-23.
Adaptations to nitrogen availability drive ecological divergence of chemosynthetic symbionts
Morel-Letelier I, Yuen B, Kück AC, Camacho-García YE, Petersen JM, Lara M, Leray M, Eisen JA, Osvatic JT, Gros O, Wilkins LG. 2024 – PLoS genetics. 20:e1011295.
Adaptation to tolerate high doses of arabinoxylan is associated with fecal levels of Bifidobacterium longum
Deehan EC, Zhang Z, Nguyen NK, Perez-Muñoz ME, Cole J, Riva A, Berry D, Prado CM, Walter J. 2024 – Gut Microbes 16:2363021.
Climate influences the gut eukaryome of wild rodents in the Great Rift Valley of Jordan
Khadem S, Berry D, Al-Khlifeh E. 2024 – Parasites Vectors. 17:358.
MicrobioRaman: an open-access web repository for microbiological Raman spectroscopy data
Lee KS, Landry Z, Athar A, Alcolombri U, Pramoj Na Ayutthaya P, Berry D, de Bettignies P, Cheng JX, Csucs G, Cui L, Deckert V. 2024 – Nat Microbiol 7:1-5.
Neuroactive metabolites and bile acids are altered in extremely premature infants with brain injury
Pristner M, Wasinger D, Seki D, Klebermaß-Schrehof K, Berger A, Berry D, Wisgrill L, Warth B. 2024 – Cell Reports Medicine 5
A metalloprotease secreted by an environmentally acquired gut bacterium hinders Borrelia afzelii colonization in Ixodes ricinus
Hodzic A, Veinovic G, Alic A, Seki D, Kunert M, Nikolov G, Sukara R, Supic J, Tomanovic S, Berry D. 2024 – Front Cell Infect Microbiol 14:1476266.
Inhibition profile of three biological nitrification inhibitors and their response to soil pH modification in two contrasting soils
Rojas-Pinzon P A, Prommer J, Sedlacek C J, Sandén T, Spiegel H, Pjevac P, Fuchslueger L, Giguere A t. 2024 – FEMS Microbiology Ecology
Salivary microbiome and MRP-8/14 levels in children with gingivitis, healthy children, and their mothers
Blufstein A, Pejcic N, Spettel K, Hausmann B, Seki D, Ertekin T, Hinrichs-Priller J, Altner S, Nehr M, Bekes K, Makristathis A, Andrukhov O. 2024 – J Periodontol, in press
Co-occurring nitrifying symbiont lineages are vertically inherited and widespread in marine sponges
Glasl B, Luter HM, Damjanovic K, Kitzinger K, Mueller AJ, Mahler L, Engelberts JP, Rix L, Osvatic JT, Hausmann B, Séneca J, Daims H, Pjevac P, Wagner M. 2024 – ISME J, in press
Global abundance patterns, diversity, and ecology of Patescibacteria in wastewater treatment plants
Hu H, Kristensen JM, Herbold CW, Pjevac P, Kitzinger K, Hausmann B, Dueholm MKD, Nielsen PH, Wagner M. 2024 – Microbiome, 1: 55
Viral potential to modulate microbial methane metabolism varies by habitat
Zhong ZP, Du J, Köstlbacher S, Pjevac P, Orlić S, Sullivan MB2024 – Nat Commun, 1: 1857
Soil warming increases the number of growing bacterial taxa but not their growth rates.
Metze D, Schnecker J, de Carlan CLN, Bhattarai B, Verbruggen E, Ostonen I, Janssens IA, Sigurdsson BD, Hausmann B, Kaiser C, Richter A. 2024 – Sci Adv, 8: eadk6295
The maternal microbiome in pregnancy, delivery, and early-stage development of neonatal microbiome after cesarean section: A prospective longitudinal study
Foessleitner P, Pjevac P, Granser S, Wisgrill L, Pummer L, Eckel F, Seki D, Berry D, Hausmann B, Farr A. 2024 – Acta Obstet Gynecol Scand, in press
Biomonitoring of Dietary Mycotoxin Exposure and Associated Impact on the Gut Microbiome in Nigerian Infants
Ayeni KI, Seki D, Pjevac P, Hausmann B, Krausová M, Braun D, Wisgrill L, Berry D, Warth B, Ezekiel CN2024 - Environ Sci Technol, in press
Obesity increases allergic airway inflammation that can be successfully treated by oral tolerance
Geissler N, Orola M, Alinaghi M, Nardo A, Stulnig TM, Séneca J, Schmid A, Korb E, Svoboda T, Garner-Spitzer E, Kundi M, Ehling-Schulz M, Schabussova I, Inic-Kanada A, Wiedermann U. 2024 – Allergy, 2: 529-533
2023
Pathometagenomics reveals susceptibility to intestinal infection by Morganella to be mediated by the blood group-related B4galnt2 gene in wild mice
Vallier M, Suwandi A, Ehrhardt K, Belheouane M, Berry D, Čepić A, Galeev A, Johnsen JM, Grassl GA, Baines JF. – Gut Microbes 15:2164448.
Fiber consumption stimulates the activity of microbial bile salt hydrolases
Gregor A, Auernigg-Haselmaier S, Malleier M, Bruckberger S, Séneca J, Pjevac P, Pignitter M, Duszka. 2023 – Journal of Functional Foods, 107:105707
Defects in microvillus crosslinking sensitize to colitis and inflammatory bowel disease
Mödl B, Awad M, Zwolanek D, Scharf I, Schwertner K, Milovanovic D, Moser D, Schmidt K, Pjevac P, Hausmann B, Krauß D, Mohr T, Svinka J, Kenner L, Casanova E, Timelthaler G, Sibilia M, Krieger S, Eferl R. 2023 - EMBO Reports, in press.
Identification of inulin-responsive bacteria in the gut microbiota via multi-modal activity-based sorting.
Riva A, Rasoulimehrabani H, Cruz-Rubio JM, Schnorr SL, von Baeckmann C, Inan D, Nikolov G, Herbold CW, Hausmann B, Pjevac P, Schintlmeister A, Spittler A, Palatinszky M, Kadunic A, Hieger N, Del Favero G, von Bergen M, Jehmlich N, Watzka M, Lee KS, Wiesenbauer J, Khadem S, Viernstein H, Stocker R, Wagner M, Kaiser C, Richter A, Kleitz F, Berry D. 2023 - Nat Commun, 1: 8210
Apelin and the gut microbiome: Potential interaction in human MASLD.
Effenberger M, Grander C, Hausmann B, Enrich B, Pjevac P, Zoller H, Tilg H. 2023 - Dig Liver Dis, in press
Microclimate shapes the phylosymbiosis of rodent gut microbiota in Jordan's Great Rift Valley.
Al-Khlifeh E, Khadem S, Hausmann B, Berry D. 2023 - Front Microbiol, 1258775.
24-Norursodeoxycholic acid ameliorates experimental alcohol-related liver disease and activates hepatic PPARγ.
Grander C, Meyer M, Steinacher D, Claudel T, Hausmann B, Pjevac P, Grabherr F, Oberhuber G, Grander M, Brigo N, Jukic A, Schwärzler J, Weiss G, Adolph TE, Trauner M, Tilg H. 2023 - JHEP Rep, 11: 100872.
The phageome in normal and inflamed human skin.
Wielscher M, Pfisterer K, Samardzic D, Balsini P, Bangert C, Jäger K, Buchberger M, Selitsch B, Pjevac P, Willinger B, Weninger W. 2023 - Sci Adv, 39: eadg4015.
Cultivation and genomic characterization of novel and ubiquitous marine nitrite-oxidizing bacteria from the Nitrospirales.
Mueller AJ, Daebeler A, Herbold CW, Kirkegaard RH, Daims H. 2023 - ISME J, in press.
Microbial growth under drought is confined to distinct taxa and modified by potential future climate conditions.
Metze D, Schnecker J, Canarini A, Fuchslueger L, Koch BJ, Stone BW, Hungate BA, Hausmann B, Schmidt H, Schaumberger A, Bahn M, Kaiser C, Richter A. 2023 - Nat Commun, 1: 5895.
Ecophysiology and interactions of a taurine-respiring bacterium in the mouse gut.
Ye H, Borusak S, Eberl C, Krasenbrink J, Weiss AS, Chen SC, Hanson BT, Hausmann B, Herbold CW, Pristner M, Zwirzitz B, Warth B, Pjevac P, Schleheck D, Stecher B, Loy A. 2023 - Nat Commun, 1: 5533.
Microbial Diversity and Activity of Biofilms from Geothermal Springs in Croatia.
Kostešić E, Mitrović M, Kajan K, Marković T, Hausmann B, Orlić S, Pjevac P. 2023 - Microb Ecol, in press.
Pitfalls in sampling and analyzing low-biomass human nasal microbiome samples.
Pjevac P, Bartosik T, Schneider S, Eckl-Dorna J. 2023 - J Allergy Clin Immunol, 1: 304
The nasal microbiome in patients suffering from non-steroidal anti-inflammatory drugs-exacerbated respiratory disease in absence of corticosteroids.
Bartosik TJ, Campion NJ, Freisl K, Liu DT, Gangl K, Stanek V, Tu A, Pjevac P, Hausmann B, Eckl-Dorna J, Schneider S. 2023 - Front Immunol, 1112345.
Hydrochemical and Seasonally Conditioned Changes of Microbial Communities in the Tufa-Forming Freshwater Network Ecosystem.
Čačković A, Kajan K, Selak L, Marković T, Brozičević A, Pjevac P, Orlić S. 2023 - mSphere, e0060222.
From the Mountain to the Valley: Drivers of Groundwater Prokaryotic Communities along an Alpine River Corridor.
Retter A, Haas JC, Birk S, Stumpp C, Hausmann B, Griebler C, Karwautz C. 2023 - Microorganisms, 3: in press.
Gut microbiome signatures of Yorkshire Terrier enteropathy during disease and remission.
Doulidis PG, Galler AI, Hausmann B, Berry D, Rodríguez-Rojas A, Burgener IA. 2023 - Sci Rep, 1: 4337.
Secondary Metabolite Production Potential in a Microbiome of the Freshwater Sponge Spongilla lacustris.
Graffius S, Garzón JFG, Zehl M, Pjevac P, Kirkegaard R, Flieder M, Loy A, Rattei T, Ostrovsky A, Zotchev SB. 2023 - Microbiol Spectr, 2: e0435322.
Gene loss and symbiont switching during adaptation to the deep sea in a globally distributed symbiosis.
Osvatic JT, Yuen B, Kunert M, Wilkins L, Hausmann B, Girguis P, Lundin K, Taylor J, Jospin G, Petersen JM. 2023 - ISME J, 3: 453-466.
The microbiome of kidney stones and urine of patients with nephrolithiasis.
Lemberger U, Pjevac P, Hausmann B, Berry D, Moser D, Jahrreis V, Özsoy M, Shariat SF, Veser J. 2023 - Urolithiasis, 1: 27.
Microbial communities and processes in biofilters for post-treatment of ozonated wastewater treatment plant effluent.
Sauter D, Steuer A, Wasmund K, Hausmann B, Szewzyk U, Sperlich A, Gnirss R, Cooper M, Wintgens T. 2023 - Sci Total Environ, 2: 159265.
2022
Individual sweet taste perception influences salivary characteristics after orosensory stimulation with sucrose and non-caloric sweeteners.
Karl CM, Vidakovic A, Pjevac P, Hausmann B, Schleining G, Ley JP, Berry D, Hans J, Wendlin M, Koenig J, Somoza V, Lieder B. 2022 - Frontiers in Nutrition, 737: 831726.
Nutrient niche specificity for glycosaminoglycans is reflected in polysaccharide utilization locus architecture of gut species.
Overbeeke A, Hausmann B, Nikolov G, Pereira FC, Herbold CW, Berry D. 2022 - Front Microbiol, 1033355.
Disturbances in microbial skin recolonization and cutaneous immune response following allogeneic stem cell transfer.
Bayer N, Hausman B, Pandey RV, Deckert F, Gail LM, Strobl J, Pjevac P, Krall C, Unterluggauer L, Redl A, Bachmayr V, Kleissl L, Nehr M, Kirkegaard R, Makristathis A, Watzenboeck ML, Nica R, Staud C, Hammerl L, Wohlfarth P, Ecker RC, Knapp S, Rabitsch W, Berry D, Stary G. 2022 - Leukemia, 11: 2705-2714.
Arbuscular Mycorrhiza and Nitrification: Disentangling Processes and Players by Using Synthetic Nitrification Inhibitors.
Dudáš M, Pjevac P, Kotianová M, Gančarčíková K, Rozmoš M, Hršelová H, Bukovská P, Jansa J. 2022 - Appl Environ Microbiol, 20: e0136922.
Microbial community composition and hydrochemistry of underexplored geothermal waters in Croatia.
Mitrović M, Kostešić E, Marković T, Selak L, Hausmann B, Pjevac P, Orlić S. 2022 - Syst Appl Microbiol, 6: 126359.
Impaired Mucosal Homeostasis in Short-Term Fiber Deprivation Is Due to Reduced Mucus Production Rather Than Overgrowth of Mucus-Degrading Bacteria.
Overbeeke A, Lang M, Hausmann B, Watzka M, Nikolov G, Schwarz J, Kohl G, De Paepe K, Eislmayr K, Decker T, Richter A, Berry D. 2022 - Nutrients, 18: in press.
How to Verify Non-Presence-The Challenge of Axenic Algae Cultivation.
Pokorny L, Hausmann B, Pjevac P, Schagerl M. 2022 - Cells, 16: in press.
Microbial marker for seawater intrusion in a coastal Mediterranean shallow Lake, Lake Vrana, Croatia.
Selak L, Marković T, Pjevac P, Orlić S. 2022 - Sci Total Environ, 157859.
Oxford Nanopore R10.4 long-read sequencing enables the generation of near-finished bacterial genomes from pure cultures and metagenomes without short-read or reference polishing.
Sereika M, Kirkegaard RH, Karst SM, Michaelsen TY, Sørensen EA, Wollenberg RD, Albertsen M. 2022 - Nat Methods, 7: 823-826.
SRS-FISH: A high-throughput platform linking microbiome metabolism to identity at the single-cell level.
Ge X, Pereira FC, Mitteregger M, Berry D, Zhang M, Hausmann B, Zhang J, Schintlmeister A, Wagner M, Cheng JX. 2022 - Proc Natl Acad Sci U S A, 26: e2203519119.
Chemoautotrophy, symbiosis and sedimented diatoms support high biomass of benthic molluscs in the Namibian shelf.
Amorim K, Loick-Wilde N, Yuen B, Osvatic JT, Wäge-Recchioni J, Hausmann B, Petersen JM, Fabian J, Wodarg D, Zettler ML. 2022 - Sci Rep, 1: 9731.
Elucidating the role of the gut microbiota in the physiological effects of dietary fiber.
Deehan EC, Zhang Z, Riva A, Armet AM, Perez-Muñoz ME, Nguyen NK, Krysa JA, Seethaler B, Zhao YY, Cole J, Li F, Hausmann B, Spittler A, Nazare JA, Delzenne NM, Curtis JM, Wismer WV, Proctor SD, Bakal JA, Bischoff SC, Knights D, Field CJ, Berry D, Prado CM, Walter J. 2022 - Microbiome, 1: 77.
Differential Modulation of the European Sea Bass Gut Microbiota by Distinct Insect Meals.
Rangel F, Enes P, Gasco L, Gai F, Hausmann B, Berry D, Oliva-Teles A, Serra CR, Pereira FC. 2022 - Front Microbiol, 831034.
Individuality of the Extremely Premature Infant Gut Microbiota Is Driven by Ecological Drift.
Seki D, Schauberger C, Hausmann B, Berger A, Wisgrill L, Berry D. 2022 - mSystems, e0016322.
Interleukin-11 drives human and mouse alcohol-related liver disease.
Effenberger M, Widjaja AA, Grabherr F, Schaefer B, Grander C, Mayr L, Schwaerzler J, Enrich B, Moser P, Fink J, Pedrini A, Jaschke N, Kirchmair A, Pfister A, Hausmann B, Bale R, Putzer D, Zoller H, Schafer S, Pjevac P, Trajanoski Z, Oberhuber G, Adolph T, Cook S, Tilg H. 2022 - Gut, in press.
How low can they go? Aerobic respiration by microorganisms under apparent anoxia.
Berg J, Ahmerkamp S, Pjevac P, Hausmann B, Milucka J, Kuypers MMM. 2022 - FEMS Microbiol Rev, epub.
2021
Reduced alpha diversity of the oral microbiome correlates with short progression‐free survival in patients with relapsed/refractory multiple myeloma treated with ixazomib‐based therapy (AGMT MM 1, phase II trial).
Ludwig H, Hausmann B, Schreder M, Pönisch W, Zojer N, Knop S, Gunsilius E, Egle A, Petzer A, Einsele H, Hajek R, Weisel K, Krenosz KJ, Lang A, Lechner D, Greil R, Berry D. 2021 - eJHaem, 2: 102–106.
Gilbert's Syndrome and the Gut Microbiota - Insights From the Case-Control BILIHEALTH Study.
Zöhrer PA, Hana CA, Seyed Khoei N, Mölzer C, Hörmann-Wallner M, Tosevska A, Doberer D, Marculescu R, Bulmer AC, Herbold CW, Berry D, Wagner KH. 2021 - Front Cell Infect Microbiol, 701109.
Aberrant gut-microbiota-immune-brain axis development in premature neonates with brain damage.
Seki D, Mayer M, Hausmann B, Pjevac P, Giordano V, Goeral K, Unterasinger L, Klebermaß-Schrehof K, De Paepe K, Van de Wiele T, Spittler A, Kasprian G, Warth B, Berger A, Berry D, Wisgrill L. 2021 - Cell Host Microbe, in press.
Mucosal Biofilms Are an Endoscopic Feature of Irritable Bowel Syndrome and Ulcerative Colitis.
Baumgartner M, Lang M, Holley H, Crepaz D, Hausmann B, Pjevac P, Moser D, Haller F, Hof F, Beer A, Orgler E, Frick A, Khare V, Evstatiev R, Strohmaier S, Primas C, Dolak W, Köcher T, Klavins K, Rath T, Neurath MF, Berry D, Makristathis A, Muttenthaler M, Gasche C. 2021 - Gastroenterology, 4: 1245-1256.e20.
Microbial community structure in hadal sediments: high similarity along trench axes and strong changes along redox gradients.
Schauberger C, Glud RN, Hausmann B, Trouche B, Maignien L, Poulain J, Wincker P, Arnaud-Haond S, Wenzhöfer F, Thamdrup B. 2021 - ISME J, in press.
An Economical and Flexible Dual Barcoding, Two-Step PCR Approach for Highly Multiplexed Amplicon Sequencing.
Pjevac P, Hausmann B, Schwarz J, Kohl G, Herbold CW, Loy A, Berry D. 2021 - Front Microbiol, 669776.
Functional iron-deficiency in women with allergic rhinitis is associated with symptoms after nasal provocation and lack of iron-sequestering microbes.
Petje LM, Jensen SA, Szikora S, Sulzbacher M, Bartosik T, Pjevac P, Hausmann B, Hufnagl K, Untersmayr E, Fischer L, Vyskocil E, Eckl-Dorna J, Jensen-Jarolim E, Hofstetter G, Afify SM, Krenn CG, Roth GA, Rivelles E, Hann S, Roth-Walter F. 2021 - Allergy, 9: 2882-2886.
Novel taxa of Acidobacteriota implicated in seafloor sulfur cycling.
Flieder M, Buongiorno J, Herbold CW, Hausmann B, Rattei T, Lloyd KG, Loy A, Wasmund K. 2021 - ISME J, in press.
Combined hormonal contraceptives are associated with minor changes in composition and diversity in gut microbiota of healthy women.
Mihajlovic J, Leutner M, Hausmann B, Kohl G, Schwarz J, Röver H, Stimakovits N, Wolf P, Maruszczak K, Bastian M, Kautzky-Willer A, Berry D. 2021 - Environ Microbiol, 6: 3037-3047.
In vitro interactions of Alternaria mycotoxins, an emerging class of food contaminants, with the gut microbiota: a bidirectional relationship.
Crudo F, Aichinger G, Mihajlovic J, Varga E, Dellafiora L, Warth B, Dall'Asta C, Berry D, Marko D. 2021 - Arch Toxicol, .
Sulfoquinovose is a select nutrient of prominent bacteria and a source of hydrogen sulfide in the human gut.
Hanson BT, Dimitri Kits K, Löffler J, Burrichter AG, Fiedler A, Denger K, Frommeyer B, Herbold CW, Rattei T, Karcher N, Segata N, Schleheck D, Loy A. 2021 - ISME J, In press.
Anaerobic bacterial degradation of protein and lipid macromolecules in subarctic marine sediment.
Pelikan C, Wasmund K, Glombitza C, Hausmann B, Herbold CW, Flieder M, Loy A. 2021 - ISME J, 3: 833-847.
2020
Conversion of Rutin, a Prevalent Dietary Flavonol, by the Human Gut Microbiota.
Riva A, Kolimár D, Spittler A, Wisgrill L, Herbold CW, Abrankó L, Berry D. 2020 - Front Microbiol, 585428.
Polyphenol Exposure, Metabolism, and Analysis: A Global Exposomics Perspective.
Oesterle I, Braun D, Berry D, Wisgrill L, Rompel A, Warth B. 2020 - Annu Rev Food Sci Technol, .
Optofluidic Raman-activated cell sorting for targeted genome retrieval or cultivation of microbial cells with specific functions.
Lee KS, Pereira FC, Palatinszky M, Behrendt L, Alcolombri U, Berry D, Wagner M, Stocker R. 2020 - Nat Protoc, -.
Rational design of a microbial consortium of mucosal sugar utilizers reduces Clostridiodes difficile colonization.
Pereira FC, Wasmund K, Cobankovic I, Jehmlich N, Herbold CW, Lee KS, Sziranyi B, Vesely C, Decker T, Stocker R, Warth B, von Bergen M, Wagner M, Berry D. 2020 - Nat Commun, 1: 5104.
A refined set of rRNA-targeted oligonucleotide probes for in situ detection and quantification of ammonia-oxidizing bacteria.
Lukumbuzya M, Kristensen JM, Kitzinger K, Pommerening-Roser A, Nielsen PH, Wagner M, Daims H, Pjevac P. 2020 - Water Res, 116372.
Composition and activity of nitrifier communities in soil are unresponsive to elevated temperature and CO, but strongly affected by drought.
Séneca J, Pjevac P, Canarini A, Herbold CW, Zioutis C, Dietrich M, Simon E, Prommer J, Bahn M, Pötsch EM, Wagner M, Wanek W, Richter A. 2020 - ISME J, 12: 3038-3053.
Gut microbiota and undigested food constituents modify toxin composition and suppress the genotoxicity of a naturally occurring mixture of Alternaria toxins in vitro.
Crudo F, Aichinger G, Mihajlovic J, Dellafiora L, Varga E, Puntscher H, Warth B, Dall'Asta C, Berry D, Marko D. 2020 - Arch. Toxicol. ,
Crypt residing bacteria and proximal colonic carcinogenesis in a mouse model of Lynch syndrome.
Lang M, Baumgartner M, Rożalska A, Frick A, Riva A, Jarek M, Berry D, Gasche C. 2020 - Int. J. Cancer, 8: 2316-2326.
Activity and Metabolic Versatility of Complete Ammonia Oxidizers in Full-Scale Wastewater Treatment Systems.
Yang Y, Daims H, Liu Y, Herbold CW, Pjevac P, Lin JG, Li M, Gu JD. 2020 - MBio, 2: e03175-19.
Single cell analyses reveal contrasting life strategies of the two main nitrifiers in the ocean.
Kitzinger K, Marchant HK, Bristow LA, Herbold CW, Padilla CC, Kidane AT, Littmann S, Daims H, Pjevac P, Stewart FJ, Wagner M, Kuypers MMM. 2020 - Nat Commun, 1: 767.
Transcriptomic Response of Nitrosomonas europaea Transitioned from Ammonia- to Oxygen-Limited Steady-State Growth.
Sedlacek CJ, Giguere AT, Dobie MD, Mellbye BL, Ferrell RV, Woebken D, Sayavedra-Soto LA, Bottomley PJ, Daims H, Wagner M, Pjevac P. 2020 - mSystems, 1: e00562-19.
The role of gut microbiota, butyrate and proton pump inhibitors in amyotrophic lateral sclerosis: a systematic review.
Erber AC, Cetin H, Berry D, Schernhammer ES. 2020 - Int J Neurosci, 7: 727-735.
2019
Draft genome sequence of Desulfosporosinus sp. strain Sb-LF, isolated from an acidic peatland in Germany.
Hausmann B, Pjevac P, Huemer M, Herbold CW, Pester M, Loy A. 2019 - Microbiology Resource Announcements, 8: e00428-19.
Draft genome sequence of Desulfosporosinus fructosivorans strain 63.6F(T), isolated from marine sediment in the Baltic Sea.
Hausmann B, Vandieken V, Pjevac P, Schreck S, Herbold CW, Loy A. 2019 - Microbiology Resource Announcements, 8: e00427-19.
2018
Draft genome sequence of Telmatospirillum siberiense 26-4b1, an acidotolerant peatland Alphaproteobacterium potentially involved in sulfur cycling.
Hausmann B, Pjevac P, Schreck K, Herbold CW, Daims H, Wagner M, Loy A. 2018 - Genome Announc, e01524-17.